Abstract
Introduction aHUS is thrombotic microangiopathy associated with excess activation of alternative complement pathway (AP). Virtually all germline mutations that predispose to aHUS [e.g., factor H (fH)] occur in genes that regulate the amplification loop in the AP. The modified Ham test (mHam) measures the ability of a nucleated cell to protect itself from complement-mediated cell death (in vitro) in the absence of downstream cell-surface complement regulators, CD55 and CD59. Thus, the mHam exposes defects in serum-based complement regulators/activators that involve the amplification loop. The mHam is useful in distinguishing aHUS from thrombotic thrombocytopenic purpura (TTP); limitations are the lack of a reliable positive control and the lack of a confirmatory assay. In order to develop a positive control for the mHam, three complement activators, cobra venom factor (CVF), lipopolysaccharide (LPS) and sialidase (neuraminidase) were studied. CVF and LPS can activate and deplete serum of complement. Sialidase (Sia) makes cells more susceptible to complement mediated killing by removing sialic acid residues that are critical for binding the complement regulator, fH. Here, we show that Sia is a reliable positive control for the mHam and that cell surface C5b-9 accumulation correlates with a positive mHam.
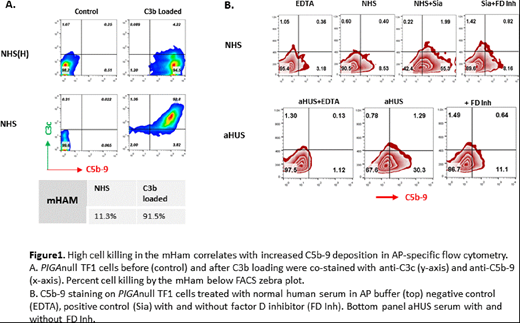
Methods/Results: The mHam assay was performed utilizing the PIGAnull TF1 cell line as previously described. CVF and LPS in dosages of 1.0 to 10ug/mL were added to 20% normal human serum (NHS) individually for 15 minutes at 37°C, and then added to cells in GVB++ buffer for 30 minutes. Sia (6.25unit/mL to 200unit/mL) was added to cells in GVB++ buffer for 15 minutes at 37°C first, and then incubated with 20% normal human serum (NHS) for 30 minutes. Complement-mediated cell killing in the mHam was evaluated using WST-1 absorbance as described previously. We found that sia treatment was more effective and reliable than LPS and CVF at inducing complement-mediated killing. A sia dose of 50U/mL or greater increased complement mediated killing by more than 3-fold in the mHam. Cell killing correlated with increased C5b-9 staining and was abrogated by the addition of an anti-C5 antibody and a factor D inhibitor. Next, we loaded PIGAnull TF1 cells with increasing amounts of C3b, added 20% NHS, and simultaneously performed the mHam and stained cells with an anti-C5b-9 monoclonal antibody for FACS staining to see if C5b-9 accumulation correlates with mHam killing. Briefly, PIGAnull TF1 cells in GVB+ buffer were incubated with 17.5ug C3, 4ug factor B and 0.1ug factor D for 20 minutes at room temperature (RT). Cells were washed and then incubated with 2ug factor B and 0.1ug factor D at RT for 3 minutes first, and treated with 0.25M EDTA in GVB0 buffer. Then 17.5ug C3 was added to cells for 15 minutes at 37°C. After three consecutive cycles of C3b loading, 20% NHS was added and complement activity was measured in the mHam and by flow cytometry. Cells loaded with one or two cycles of C3b showed minimal C5b-9 staining and minimal cell death (<15%) in the mHam; however, after three cycles there was a marked increase in C5b-9 staining (Figure 1A) that correlated with increased killing in the mHam. The addition of 2.5uM factor D inhibitor to NHS blocked C5b-9 accumulation on the PIGAnull TF1 cells. Based on these data we hypothesized that serum from aHUS patients would lead to an increase in C5b-9 staining on PIGAnull TF1 cells and also correlate with increased cell killing in the mHam. For these studies, 20% NHS from healthy controls, and aHUS was incubated with TF1PIGAnull cells. The mHam was performed as above using GVB++ buffer. For flow cytometry experiments AP pathway buffer (Mg++EGTA) was used and cells were stained with anti-C5b-9 antibody and analyzed by FACs. TF1PIGAnull cells treated with 50U/mL sialidase incubated with NHS was used as a positive control. aHUS serum from patients (with and without germline APC mutations) resulted in a marked increase in cell surface C5b-9 staining in AP buffer and correlated with increased cell death in the mHam. C5b-9 staining was blocked by addition of a factor D inhibitor (Figure 1B).
Conclusion We describe an AP pathway specific flow cytometric assay that correlates with the mHam and may further aid in the diagnosis of aHUS. Sialidase treatment of TF1PIGAnull cells serves as a reliable positive control for the mHam by making cells more susceptible to AP killing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal